Discontinued:
Doty Susceptibility-Matched Sample Plugs
for High Resolution Liquids NMR
(Wide selection of susceptibility-matched sample plugs for all NMR tubes)
|
|
Triple the Sensitivity in Sample-Limited Applications
Eliminate Convection Artifacts in Large-Sample NMR
Limited sample? Triple the relative sensitivity (S/N per unit volume) by using our susceptibility-matched sample plugs, precisely machined from chemically resistant dielectrics with low rf loss.
HR-NMR has normally required a sample length three to five times that of the active coil length because it is generally impossible to shim out the effects of a magnetic susceptibility discontinuity at a material interface (air-sample, or glass- sample) that is not parallel to B0. The greater the susceptibility discontinuity, the further it must be from the sensitive region. Plugs matched to the solvent’s susceptibility within 5% eliminate the need for 80% of the excess sample and allow sensitivity to be tripled (because of higher concentration) with no loss in resolution. Moreover, lineshape and resolution in older probes are dramatically improved.
Doty’s design solves problems with: bubble removal, glass breakage, sample length adjustment, backgrounds, and VT.
An important, new application for these plugs occurs for large samples of low solubility. Thermal gradients as small as 0.2°C in larger non-spinning sample tubes (8 mm to 15 mm) can drive sample convection and may cause serious spectral artifacts[1]. Susceptibility-matched plugs eliminate these artifacts and permit VT HR-NMR on large, non-spinning samples.
Plug Materials: Susceptibility and Chemical CompatibilitySelect plug material with magnetic susceptibility similar to that of the sample solvent. Select Kel-F or zirconia when wideline 1H backgrounds would cause problems |
Solvent |
Suscept. | ||||||||
|
C3H8O3 |
0.779 |
|||||||||
|
– |
Kel-F |
(reference) Pyrex |
PPS |
Aurum |
Ultem |
Zirconia |
GFP |
G-10 |
C6H5Br |
0.753 |
|
– χVC (ppm, cgs) |
0.92 |
0.86 |
0.73 |
0.71 |
0.71 |
0.70 |
0.52 |
~ 0.5 |
CHCl3 |
0.74 |
|
Wideline NMR Backgrounds |
F, Cl, C |
Si, B, Al, Na |
H, C, S |
H, C, N |
H, C, N |
Zr |
H, C, Al, Si, F |
H, C, Al, Si, F |
H2O |
0.719 |
|
H2O absorp. % |
0.02 |
0.01 |
0.03 |
0.8 |
0.7 |
0.01 |
0.2 |
0.15 |
D2O |
0.70 |
|
Density, g/cm3 |
2.1 |
2.5 |
1.35 |
1.42 |
1.27 |
5.7 |
1.45 |
1.88 |
CS2 |
0.70 |
|
Max use T, |
150 |
400 |
120 |
240 |
205 |
700 |
250 |
160 |
CCl4 |
0.691 |
|
Color |
clear |
glass |
ivory |
black |
amber |
white |
grey |
green |
C8H16 |
0.684 |
|
Chemical Resistance: E- Excellent; G- Good, usually acceptable; F- Fair, sometimes acceptable; P- Poor |
C6H12 |
0.627 |
||||||||
|
C7H8 |
0.618 |
|||||||||
|
Strong acids |
E |
E |
G |
G |
G |
E |
P |
F |
C6H6 |
0.611 |
|
Strong bases |
E |
E |
E |
G |
G |
E |
G |
E |
C6H5NO2 |
0.604 |
|
Alcohols & Aliphatics |
E |
E |
E |
E |
E |
E |
E |
E |
C3H8O |
0.598 |
|
Aromatic H-C |
E |
E |
E |
E |
G |
E |
E |
E |
C2H6O |
0.575 |
|
Esters & Ketones |
E |
E |
E |
E |
E |
E |
E |
E |
(C2H5)2O |
0.534 |
|
Chloro-solvents |
E |
E |
E |
G |
F |
E |
G |
G |
CH4O |
0.53 |
|
Volumetric suscept. χV in S.I. is related to vol. suscept. χVC in cgs by: χV= 4 πχVC |
(CH3)2CO |
0.46 |
||||||||
[1] J. Lounila et al, J. Magn. Reson. Ser. A , 118 , 1996.
Abbreviations: Kel-F , p-chlorotrifluoroethylene; G-10 , 60% e-glass epoxy composite; PPS , p-phenylene sulfide; Aurum , thermoplastic polyimide; Ultem , p-etherimide; GFP , glass-filled PEEK (p-etheretherketone) ; CPVC , Chlorinated-p-vinylchloride.
|
Solvent |
Solvent-χ(cgs) |
Plug Material |
Plug -χ |
% H2O absorp. |
Comments |
|
Glycerol |
0.78 |
Kel-F |
0.92 |
0.02 |
Kel-F is often selected for many other solvents because it is proton-free and has superb solvent resistance. |
|
Chloro- form |
0.74 |
PPS* |
0.73 |
0.03 |
PPS is also sometimes preferred for water and many other solvents. PPS is resistant to all known solvents except the strongest acids. |
|
Water |
0.72 |
Ultem |
0.71 |
0.70 |
Ultem is a low-cost, disposable option for water and D2O but its resistance to chloro-solvents and aromatics may be inadequate. |
|
D2O |
0.70 |
Aurum |
0.71 |
0.80 |
Aurum , a polyimide, is often the best choice for water, except where water suppression is critical. Aurum is also preferred for most solvents at high temperatures. |
|
Carbon tetrachl. |
0.69 |
Zirconia |
0.70 |
0.01 |
Zirconia is also preferred for cyclohexane, toluene, benzene and for most wide-line proton applications. |
|
Methyl alcohol |
0.53 |
GFP |
0.52 |
0.20 |
Glass-filled PEEK is also preferred for ethyl ether and usually ethyl alcohol. |
|
Acetone |
0.46 |
G-10 |
~0.5 |
0.15 |
G-10 is also a low-cost option for methyl alcohol. |
* The lower plug contains an imbedded weight to keep it from floating in dense solvents.
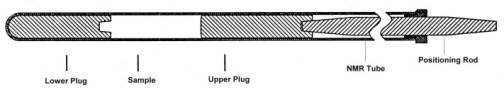
Using Doty Susceptibility Plugs
Loading
• Add the desired amount of sample to the NMR tube
• Drop in the lower plug with the threaded hole up and filled with sample. Use a rod to push it all the way down (snapping it quickly into the bottom helps clear bubbles)
• Screw* an upper plug onto the end of an adjustment rod
• Slide a clamp ring over the end of the rod
• Drop the upper plug and rod into the NMR tube
• Rotate and jostle the plug to clear bubbles (vent grooves help with high-viscosity solvents)
• Slide the clamp ring for the desired sample length (upper plug must remain covered with at least 1 mm of sample)
• Adjust the spinner turbine to center sample within rf coil
To remove the lower plug
• Lightly screw* an adjustment rod into the threaded hole on the top and pull out plug.
*Note: For zirconia plugs, the tapered end of the rod must be pressed into the plug instead of screwing the rod in place.
Distributed by Wilmad-LabGlass
Phone 1-800-220-5171
Phone 856-691-3200
Fax 856-205-1984

